You’ve probably heard of PRISMA in the context of reporting guidelines for systematic reviews. But what exactly is it and why is it so important? And what changed in the updated PRISMA 2020 guideline 🤔 ?
Clear reporting in systematic reviews is essential. It gives readers the information to form their own views about how well a review was carried out and how applicable the findings are to their own setting. It also makes the research replicable – one of the defining features of a systematic review.
PRISMA stands for Preferred Reporting Items for Systematic Reviews and Meta-analyses. Research by Cynthia Mulrow in the 1980s found major deficiencies in the quality of review reports. This work was the start of an initiative to improve standards in reporting that led an international group of experts to create PRISMA.
Decades later, systematic review authors everywhere use the PRISMA statement to make sure that their research is as useful as possible for patients, healthcare professionals, and for their fellow researchers 🙌 .
The PRISMA statement
The PRISMA statement is made up of a checklist and a flow diagram:
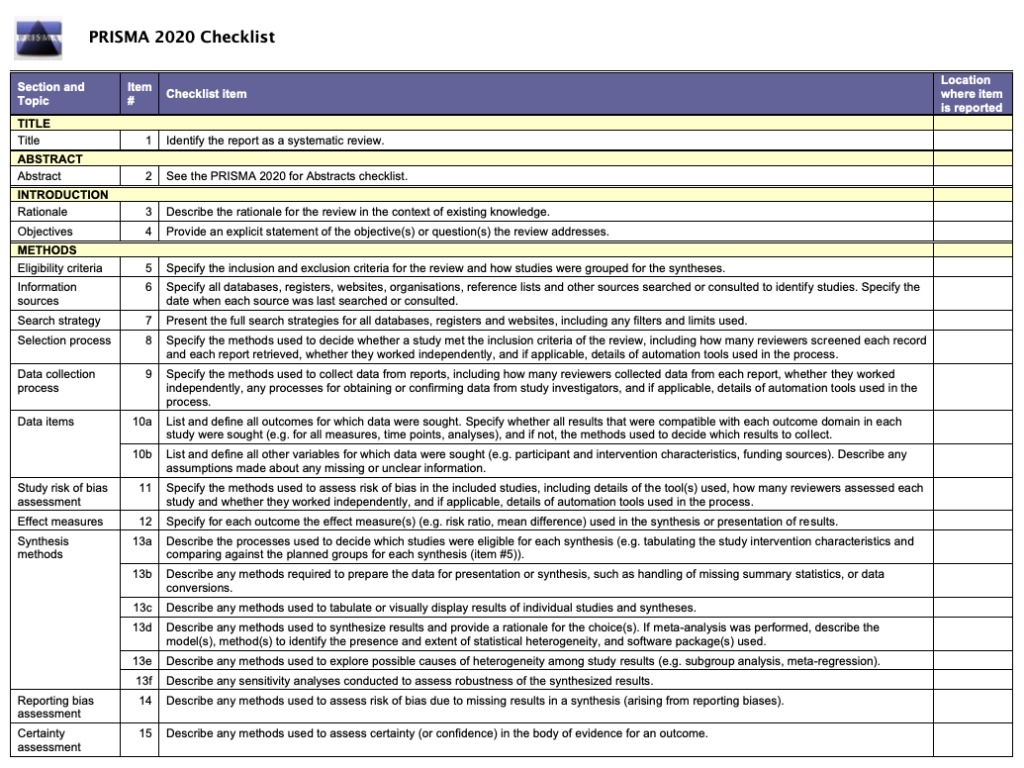
☑️ The checklist (see figure 1) sets out the 27 items that must be reported in every review. The items can be checked off the list as they are completed.
➡️ The flow diagram reports the decisions that the review team took as they assessed citations for possible inclusion in the review. It relates to checklist item #16a: “Describe the results of the search and selection process, from the number of records identified in the search to the number of studies included in the review, ideally using a flow diagram.”
The PRISMA statement is accompanied by several extensions. These are additional checklists that cover specific types or aspects of systematic reviews: protocols, searching, and scoping reviews, for example. The statement is supported by an explanation and elaboration, which gives the rationale for each checklist item along with useful examples.
Background to PRISMA 2020
First published in 2009, the PRISMA statement soon became a valued and highly cited resource for authors, journal editors, and peer reviewers alike.
PRISMA 2020 reflects more than a decade of user experience and advances in systematic review methods. The authors also hope the changes will improve the uptake of the guideline among researchers. To explain how the updated guideline was developed, it seems fitting to *checks notes* report the methods 😉.
Ok, here goes:
The PRISMA team conducted a literature review, surveyed systematic review methodologists and editors, met in person, and collaborated on multiple drafts until everyone approved the content and wording of the updated guideline.
Very meta, right? On a more serious note, Page and colleagues did a much better job of reporting these methods, as we might expect.
Before we take a look at what’s new in the updated guideline, the PRISMA 2020 statement also has a lot of useful advice for review teams, including these tips:
- Refer to the guideline early in the writing process to make sure that information is gathered on all of the items and that nothing is missed ✍🏾.
- Consider presenting detailed descriptions of methods in supplementary files, rather than in the review itself 📂.
What's new in the updated PRISMA guideline?
The PRISMA 2020 checklist still has 27 items. These correspond to the items in the previous checklist. The wording of the items has been revised for clarity and some of them now contain sub-items that reflect advances in methods since the publication of the 2009 checklist.
PRISMA 2020 has extra guidance on synthesis and assessing certainty in the body of evidence. It also has guidance on the use of automation tools, competing interests of review authors, and the availability of data.
The main changes are as follows:
- Abstract reporting checklist: previously an extension, this is now in the main guideline.
- Methods section: The ‘Protocol and registration’ item has moved to a new ‘Other’ section and a sub-item has been added.
- Methods section: The ‘Search’ item now recommends that authors present full search strategies for all databases.
- Methods section: The ‘Selection process’ item now emphasises the reporting of how many reviewers screened each record and each report retrieved, whether they worked independently and details of any automation tools used.
- Methods section: ‘Data items’ now includes a sub-item (#10a).
- Methods section: ‘Synthesis methods’ now includes six sub-items (#13a–13f).
- Results section: ‘Study selection’ now includes a sub-item (#16b).
- Results section: ‘Results of syntheses’ now includes four sub-items (#20a–20d).
- Methods section and Results section: New items recommend reporting the methods for and results of an assessment of certainty (or confidence) in the body of evidence for an outcome (#15 and #22).
- Other information: A new item recommends that authors declare any competing interests (#26).
- Other information: A new item recommends that authors state whether data used in the review are publicly available and if so, where they can be accessed (#27).
PRISMA 2020 also has an updated flow diagram template. It has optional boxes that are applicable for review updates and reviews that identify new studies via methods other than searching databases and registers. To reflect this, the PRISMA website now has four variations of the flow diagram template to choose from. The checklist and the flow diagram can be completed using new apps.
Using PRISMA in Covidence
Covidence automatically populates a PRISMA flow diagram for a review. Review teams can access their flow diagram at any time from the Review Summary page and easily export their data. As review teams import and screen references, Covidence automatically updates the flow diagram.
Find out more
If you’d like to browse other reporting guidelines, check out the EQUATOR network’s Library for health research reporting.
Save up to 71 hours on your systematic review
The #1 systematic review software.
[1] Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. doi: 10.1136/bmj.n71
[2] Page MJ, McKenzie JE, Bossuyt PM, et al. Updating guidance for reporting systematic reviews: development of the PRISMA 2020 statement. Journal of Clinical Epidemiology 2021;134:103-112. doi:10.1016/j.jclinepi.2021.02.003



