Covidence checks the boxes on PRISMA
A good systematic reviews report their methods carefully. They apply the same care to reporting the findings of the primary studies and assessing their quality. Clear reporting is essential because it gives readers the information to form their own views about how well the review was carried out. It also makes it replicable – one of the defining characteristics of a systematic review.
Reporting standards have come a long way in recent years and that’s thanks, at least in part, to the PRISMA guidelines. New reviewers quickly become familiar with its 27-point checklist and flow diagram that shows the progress of information through the review. PRISMA is a valuable tool for reviewers who want to make sure their reporting is transparent and thorough 📋.
The problem is a complex one
The more information we accumulate, the harder it is to manage. Atul Gawande called this “the problem of extreme complexity”. “The volume and complexity of what we know has exceeded our individual ability to deliver its benefits correctly, safely, or reliably,” he wrote in The Checklist Manifesto. Systematic reviewers everywhere agreed with him.
Checklists guide us through the small but necessary things that need to be done every time we go through a particular process. They are a simple response to the idea that familiarity breeds negligence. We all need reminders for the most basic tasks because, without them, our brains quickly wander off to more interesting matters *stares out of window, wonders what’s for dinner 🍽️🤔.
So, yes, PRISMA aims to ensure reporting is done clearly and consistently. But perhaps most importantly, it helps to make sure that the most fundamental items of reporting are done at all.
Let’s take a look at PRISMA in more detail to see how it can help you and your team to produce excellent reporting ✨😀.
The concept
PRISMA stands for Preferred Reporting Items for Systematic Review and Meta-analyses. Research by Cynthia Mulrow in the 1980s found major deficiencies in the quality of review reports. This work was the start of an initiative to improve standards in reporting that led an international group of experts to create PRISMA.
PRISMA is essentially a standard operating procedure for reporting that covers a minimum set of items that must be presented in the review. The focus is on reviews that evaluate randomized trials, but PRISMA can be applied to many other types of research too.
PRISMA imposes order on what might otherwise be an overwhelming number of tasks and a huge volume of information. We often refer to the ‘PRISMA guidelines’. In fact, PRISMA is a collection of the following resources:
- the PRISMA statement (this is made up of a checklist and a flow diagram)
- the PRISMA extensions (these cover specific types or aspects of systematic reviews).
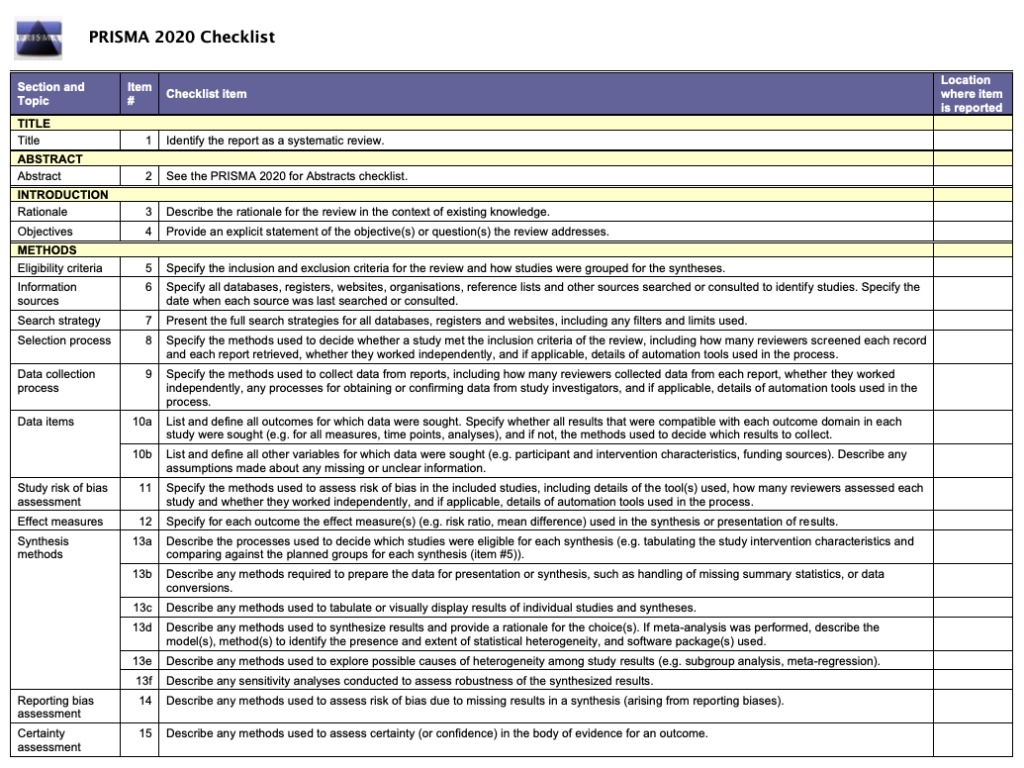
The PRISMA statement
The PRISMA statement, originally published in several journals in 2009 and updated in 2020, consists of a checklist and a flow diagram. It was written by experienced authors and methodologists with the aim of improving the quality of reporting in systematic reviews in healthcare. The accompanying explanation and elaboration provides the meaning and rationale for each of the checklist items.
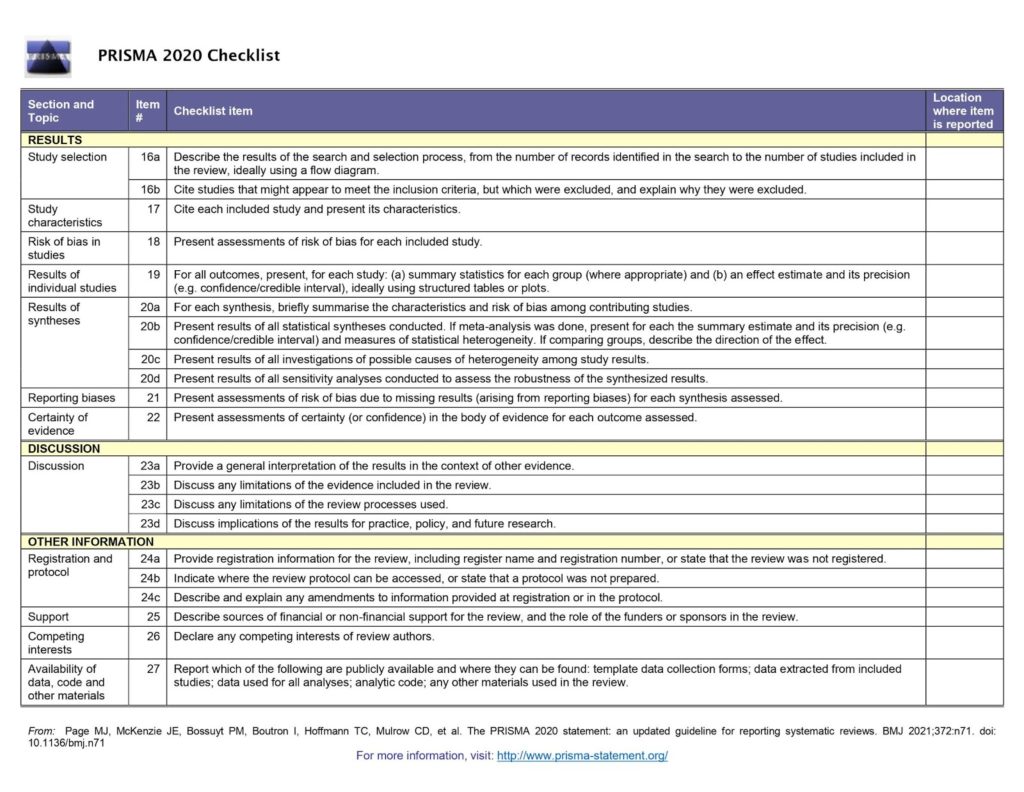
The PRISMA checklist
The PRISMA checklist covers every section of a systematic review (see figure 1). From the review title through to the methods, results and discussion, the checklist briefly sets out the 27 items that must be reported in every review. The items can be checked off (everyone’s favourite part of using a checklist 😍) as they are completed and the page number of the review added for ease of reference.
The PRISMA flow diagram
The PRISMA flow diagram relates to item 16a on the checklist:
“Describe the results of the search and selection process, from the number of records identified in the search to the number of studies included in the review, ideally using a flow diagram..”
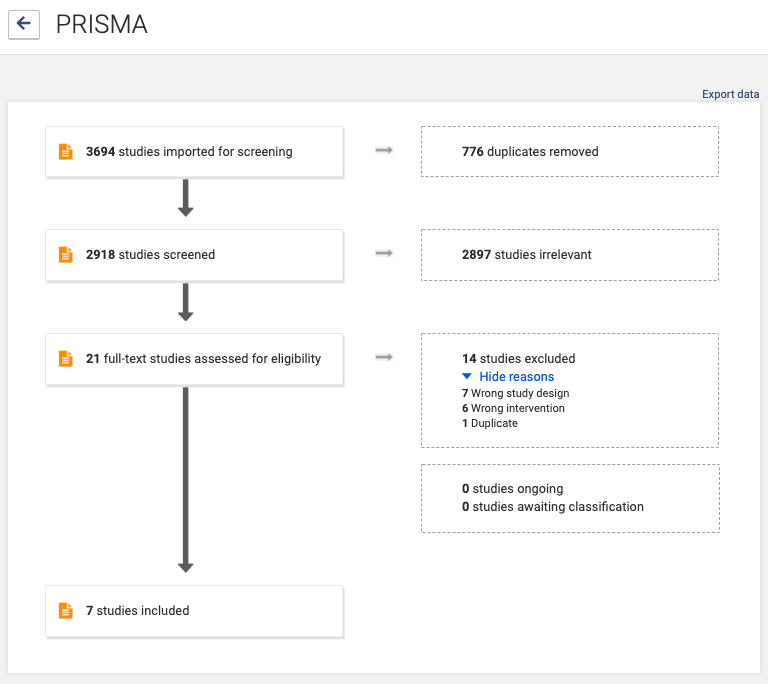
Reporting all the decisions taken around the inclusion and exclusion of studies can quickly get complicated, particularly when relying on notes produced weeks or months earlier. The PRISMA flow diagram reports the decisions that the review team took as they assessed citations for possible inclusion in the review.
The flow diagram is divided into the separate phases of the selection process: from the identification of studies by the search, through to the title and abstract screening, and the eligibility assessment based on the full text. Within these phases, the diagram sets out each action and the number of studies excluded as a result of the action until only the included studies remain. The flow diagram also includes the reasons for study exclusion, which are based on the pre-specified criteria.
The flow diagram breaks down the reporting of study selection to its most basic items and conveys the necessary information quickly and efficiently as a graphic (see figure 2). A template for the flow diagram is provided in the PRISMA statement. Covidence generates PRISMA flow diagrams automatically as part of its screening workflow, with no extra work needed 🙌.
The extensions
The PRISMA group has produced several additional checklists, called extensions. The extensions supplement the main checklist and are specific to a certain aspect, section, or type of review. The extensions cover abstracts, equity, reviews of harm outcomes, individual patient data, network meta-analysis, protocols, diagnostic test accuracy, scoping reviews, acupuncture, and searching. Extensions for newborn and child health research are under development.
Conclusion
Poor reporting can undermine good research. PRISMA provides useful evidence-based tools to ensure clear reporting in every part of a systematic review. The PRISMA flow diagram is fully integrated into Covidence and it can be accessed and updated at any time from the review summary page. Sign up for a free trial of Covidence today!